Post Herpetic Neuralgia “News”… As it Happens!
Xenon Pharmaceuticals Outlines Key Milestones for 2017?
January 8, 2017
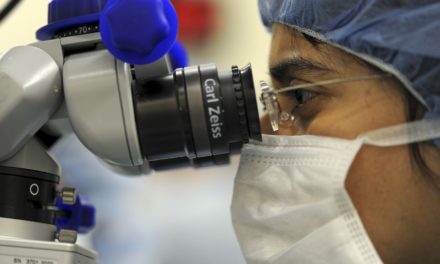
Dr. Simon Pimstone, Xenon’s President and Chief Executive Officer, said, “As we enter 2017, we anticipate our collaborator, Genentech, will advance its Nav1.7 pain program into Phase 2; and, we look forward to a topline data read-out from the Phase 2b clinical trial of TV-45070 in post-herpetic neuralgia being conducted by our collaborator, Teva.
TV-45070 is a topical sodium channel inhibitor being developed in collaboration with Xenon’s partner, Teva Pharmaceutical Industries Ltd., for the treatment of neuropathic pain. Teva is currently conducting a randomized, double-blind, placebo-controlled Phase 2b clinical trial of TV-45070 in patients with post-herpetic neuralgia, with topline results expected in mid-2017.
Did you know that:
Six out of every ten PHN sufferers who try PainBreak, a new topical analgesic,
are getting relief… Now it’s your turn!
But will PainBreak work for you? There is only one way to find out…
Complete the following “Quality of Life Questionnaire” and I will send you a 30 day supply of Painbreak absolutely “free” and postage paid.
From Boyce N Berkel, MD, PhD – Neuralgia Relief Center – For Your Health and Betterment